PREP MATERIAL
Stage 4
Acute myeloid leukemia (AML) is a malignant, biologically diverse disease characterized by proliferation of myeloid blasts in bone marrow and blood.1 In the United States, this disease is diagnosed in 2.4 per 100,000 adults and it occurs most frequently in the elderly with an incidence of 12.6 per 100,000 adults >65 years of age.2 AML in the elderly is a distinct entity that is associated with higher levels of chemotherapy resistance resulting in poor outcomes. Notably, among older AML patients, when treated with conventional dose chemotherapy, only 40-50% achieve complete remission (CR) with a long-term survival rate of <10%3,4 These response rates are significantly lower than those in younger patients who achieve CR in 70-80% and long-term survival in 30-40% of the cases. Differences in outcome between these two age groups are likely related to distinct mechanisms of leukemogenesis and overrepresentation of poor prognostic risk factors in elderly AML.2-5
Among a variety of clinical and biological factors that have been shown to predict outcome in AML, the presence of specified structural and numerical chromosomal aberrations has been identified as one of the most important to predict attainment of CR, relapse risk (RR) and overall survival (OS).6-11
Figure 1. Outcome of Older AML Based on Age Groups.
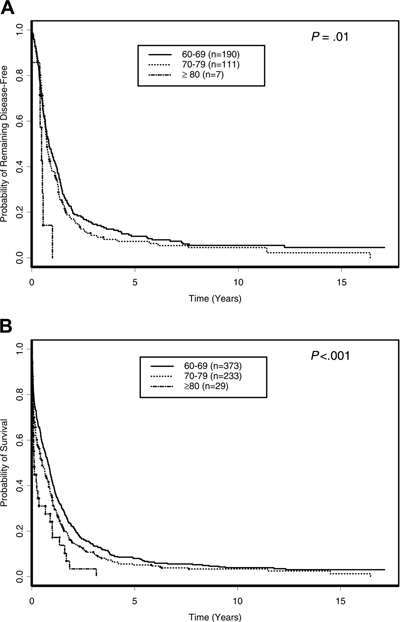
A. Disease-free survival; B. Overall survival
Farag, S. S. et al. Blood 2006;108:63-73.
Chromosome abnormalities involving genes that encoded the core binding factor (CBF) include t(8;21)(q22;q22) and inv(16) (p13q22) and have been associated with enhanced responses to chemotherapy and favorable outcomes. Acute promyelocytic leukemia, a biologically distinct form of AML characterized by the t(15;17) (q22;q11-12) translocation, has also been demonstrated to be associated with high rates of durable remission in response to combined therapy with differentiating agents such as all-trans retinoic acid (ATRA) and chemotherapy. Altogether, patients exhibiting these chromosomal patterns are considered at favorable cytogenetic risk. In contrast, patients exhibiting the -5/del(5q), -7/del(7q),inv(3)/t3;3) or complex chromosomal aberrations demonstrate intrinsically resistant disease and few experience durable responses to chemotherapy.
These abnormalities may also be found in patients with underlying myelodysplastic syndrome that subsequently evolves towards AML, a circumstance more common in elderly patients. Patients with these chromosomal aberrations are considered in the poor risk group. Included in this prognostic group are also patients with secondary AML arising from prior exposure to cytotoxic agents. Prior exposure to alkylating agents is indeed associated with the development of AML that characteristically occurs 5-7 years following therapy and may be associated with complex cytogenetics or deletions at chromosome 5 or 7. In contrast, AML may be induced by topoisomerase II inhibitors, which are associated with abnormalities in chromosome 11q23 and may develop approximately 1 year following therapy. Patients without identifiable chromosomal aberrations are considered to be in the intermediate risk category. It is important to note that older age remains a negative prognostic factor, independent of the cytogenetic risk category.
In patients with cytogenetically normal (CN) AML and those with abnormalities effecting the CBF, the presence of alterations in specific genes via mutations or aberrant expression levels has also been correlated with outcome.12,13
Figure 2. Incidence and Association of Different Molecular Aberration in Cytogenetically Normal AML.

Most of the rates refer to patients younger than 60 years.
Mrózek, K et al. Blood 2007; 109;431-448.
References
- Estey E, Döhner H. Acute myeloid leukemia. Lancet. 2006; 25;368:1894-907.
- Bowen, D.T., Etiology of acute myeloid leukemia in the elderly. Semin Hematol. 2006. 43: 82-8.
- Estey, E.H., General approach to, and perspectives on clinical research in, older patients with newly diagnosed acute myeloid leukemia. Semin Hematol. 2006. 43: 89-95.
- Buchner, T., et al., Treatment of older patients with AML. Crit Rev Oncol Hematol. 2005. 56: 247-59.
- Burnett, A.K. and U. Mohite, Treatment of older patients with acute myeloid leukemia--new agents. Semin Hematol. 2006. 43: 96-106.
- Mrózek K, Heerema NA, Bloomfield CD. Cytogenetics in acute leukemia. Blood Rev. 2004;18:115-136.
- Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92:2322-33.
- Byrd JC, Mrózek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002;100:4325-4336.
- Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group study. Blood. 2000;96:4075-4083.
- Grimwade, D., et al., The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001; 98:1312-20.
- Farag SS, Ruppert AS, Mrózek K, et al. Outcome of induction and postremission therapy in younger adults with acute myeloid leukemia with normal karyotype: a Cancer and Leukemia Group B study. J Clin Oncol. 2005;23:482-493.
- Mrózek K, Marcucci G, Paschka P, Whitman SP, Bloomfield CD. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: are we ready for a prognostically prioritized molecular classification? Blood. 2007;109:431-48.
- Marcucci, G., K. Mrózek, and C.D. Bloomfield, Molecular heterogeneity and prognostic biomarkers in adults with acute myeloid leukemia and normal cytogenetics. Curr Opin Hematol. 2005. 12: 68-75.