The Weathering Hypothesis of Aging: Part III
Course Authors
Bruce S. McEwen, Ph.D.
Dr. McEwen reports no commercial conflict of interest.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Learning Objectives
Upon completion of this Cyberounds®, you should be able to:
Discuss how the region of the brain called the hippocampus is plastic and capable of being remodeled by hormones and experience in the adult
Describe processes by which stress can remodel neuron number and dendritic branching in the hippocampus
Explain the regulation of synapse formation by estrogens in the female hippocampus.
Introduction
One of the big surprises from recent research on the response of the hippocampus to stress and stress hormones is that the hippocampal formation of an adult animal, consisting of the Ammon's horn and the dentate gyrus see Figure 1), is a dynamic brain organ in which synapses are remodeled.
Figure 1. Basic Circuitry of the Hippocampus.
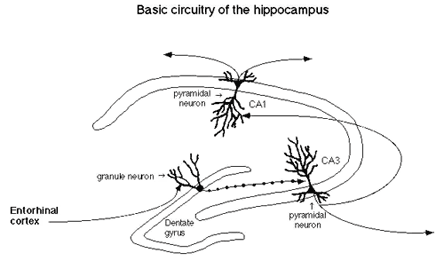
Dendrites grow and retract and dentate gyrus neurons are replaced in response to experiences.
At first, it was thought that glucocorticoids acted alone in mediating the effects of stress on hippocampal structure. Now, there is new information down-playing the relative importance of adrenal steroids as opposed to excitatory amino acids and other modulators, including neurotrophins and calcium ions.
Another aspect of hippocampal plasticity is that estrogens affect the adult hippocampus and cause the formation of synapses during the estrous cycle of a female rat. These findings have stimulated considerable interest in the beneficial effects of estrogens in the aging brain and as a possible protective agent in relation to Alzheimer's disease. This Cyberounds® discusses the plasticity of the hippocampal formation from the standpoint of the underlying mechanisms by which both estrogens and glucocorticoids have their effects.
There Are Multiple Mechanisms of Plasticity
The hippocampus is not only a vulnerable brain structure, subject to damage produced by seizures, ischemia and head trauma, but its vulnerability is also an indication that the hippocampus is a very plastic region of the brain. Adrenal steroids, which have a bad reputation as far as their role in exacerbating these forms of damage,(1) are also involved in three types of adaptive plasticity in the hippocampal formation.
First, they reversibly and biphasically modulate excitability of hippocampal neurons, influence the magnitude of long-term potentiation, as well as produce long-term depression.(2),(3),(4),(5),(6),(7) These effects may be involved in biphasic actions of adrenal secretion on excitability and cognitive function and memory during the diurnal rhythm and after stress.(8),(9),(10),(11) In particular, while acute non painful novelty stress inhibits primed-burst potentiation and memory,(12),(13) adrenal steroids also facilitate excitability and memory processes.(8),(9),(10),
Second, adrenal steroids participate, along with excitatory amino acids, in regulating neurogenesis of dentate gyrus granule neurons,(14) in which acute stressful experiences can suppress the ongoing neurogenesis.(15),(16) We believe that these effects may be involved in fear-related learning and memory, because of the anatomical and functional connections between the dentate gyrus and the amygdala,(17) a brain area important in memory of aversive and fear-producing experiences.(18)
Third, adrenal steroids participate, along with excitatory amino acids, in a reversible stress-induced atrophy, or remodelling, of dendrites in the CA3 region of the hippocampus of male rats(19) and tree shrews,(20) a process that affects only the apical dendrites and results in cognitive impairment in the learning of spatial and short-term memory tasks.(19) Although this type of plasticity does impair cognitive function, at least temporarily, it may be beneficial to the brain in the long run, if the remodelling of dendrites reduces the impact of excitatory amino acids and glucocorticoids in causing more permanent damage. This hypothesis remains to be rigorously tested.
Aside from the stress induced changes to hippocampal structure, there are other forms of plasticity in the hippocampus, including reversible synaptogenesis, that are regulated by ovarian steroids and excitatory amino acids via one type of excitatory amino acid receptor, the N-methyl-D-aspartate (NMDA) receptors. Estrogens and progestins regulate synapse formation and turnover in female rats in the CA1 region of the hippocampus via a mechanism requiring active NMDA receptors.(21),(22),(23)
There is also a reversible atrophy of dendrites of CA3 hippocampal neurons during hibernation in ground squirrels and hamsters.(24),(25) The estrogen-regulated CA1 synaptic plasticity is a rapid event occurring during the female rats' five-day estrous cycle. The synapses take several days to be induced under the influence of estrogens and endogenous glutamic acid, and then disappear within 12h under the influence of the proestrus surge of progesterone.(26) The effect of hibernation develops as rapidly as the hibernating state and recovery from hibernation takes only a few hours (24, 25; Magarinos, McEwen and Pevet, unpublished), which is one of the most rapid types of structural plasticity every documented.
In contrast, the CA3 atrophy found in rats, noted in the preceding paragraph, is a relatively slow process, taking normally at least three weeks to develop under daily stress and a week or so to disappear. However, dendritic atrophy in hibernating ground squirrels and hamsters develops as fast as the hibernating state and can be reversed rapidly within several hours (24,25; Magarinos, McEwen and Pevet, unpublished). Although anatomically similar to the stress-induced atrophy in rats and tree shrews, it is not yet clear if this process involves the same mechanisms; however, if this is the case, the question becomes what factors make the atrophy process so rapid in hibernation and slow in the case of repeated stress?
Glucocorticoids and Estrogens Do Not Work Alone
Many of the above-mentioned hormone effects on morphology and function of the hippocampus do not occur alone but rather in the context of ongoing neuronal activity. In particular, excitatory amino acids and NMDA receptors play an important role in the adaptive functional and structural changes produced in the hippocampal formation by steroid hormones. This includes not only the estradiol-induced synaptogenesis but also the effects of adrenal steroids to produce atrophy of CA3 pyramidal neurons,(19) as well as the actions of adrenal steroids to contain dentate gyrus neurogenesis.(14) Blocking NMDA receptors blocks atrophy as well as estrogen-induced synaptogenesis.(27),(28) NMDA receptors are induced by estrogens on CA1 neurons(29) and by glucocorticoids throughout the hippocampus.(30) At the same time, excitatory amino acids and NMDA receptors are involved in the destructive actions of stress and trauma on the hippocampus.(1) One of the challenges for future research is to understand what triggers the transition from adaptive plasticity to permanent damage.
With regard to the remodelling of dendrites in the CA3 region, the role of glucocorticoids is complex. Glucocorticoid treatment, using both injections or drinking water application, causes dendritic remodelling/atrophy. Stress-induced atrophy of these same dendrites is blocked by treatment with an adrenal steroid synthesis blocker, cyanoketone (see 19), indicating a role of endogenous glucocorticoids in stress-induced dendritic atrophy.
There appear to be several ways in which glucocorticoids affect the excitatory amino acid system and thus regulate this form of plasticity. First, adrenal steroids modulate expression of NMDA receptors inhippocampus,(31),(32) with chronic glucocorticoid exposure leading to increased expression of NMDA receptor binding and both NR2A and NR2B subunit mRNA levels.(30) It should be noted that the NMDA receptor is made up of subunits (NR2A and 2B) and that these subunits confer on the NMDA receptor some of its ability to be regulated by other neurochemicals.
Second, there are glucocorticoid effects on the expression of mRNA levels for specific subunits of GABAa receptors in CA3 and the dentate gyrus; both low and high levels of glucocorticoids have different effects on GABAa receptor subunit mRNA levels and receptor binding(33) suggesting that corticosterone may alter the excitability of hippocampal neurons through regulation of GABAa receptor expression. However, it remains to be seen if the corticosteroid effects on neuronal morphology involve changes in the number or pharmacological properties of GABAa receptors.
Third, adrenal steroids regulate the release of glutamate, an excitatory amino acid (EAA), since adrenalectomy markedly reduces the magnitude of the EAA release, evoked by restraint stress.(34),(35) Mossy fiber terminals in the stratum lucidum contain presynaptic kainate receptors that positively regulate glutamate release;(36) these presynaptic kainate receptors are decreased in density by ADX and restored to normal by corticosterone replacement.(37) Moreover, repeated stress causes a reorganization of synaptic vesicles within mossy fiber terminals, as reported recently using electron microscopy.(38) Whereas mossy fiber terminals (MFT) from control rats were packed with small, clear synaptic vesicles, terminals from rats receiving 21 days of restraint stress (but not after a single stress session) showed a marked rearrangement of vesicles, with more densely packed clusters localized in the vicinity of active synaptic zones. Moreover, compared with controls, restraint stress increased the area of the mossy fiber terminal occupied by mitochondrial profiles, which implies a greater, localized energy-generating capacity. Experiments are underway to find out if the primary effect of repeated stress on the mossy fiber terminals is to make them into more, rather than less, efficient synapses.
Conclusions
The dynamic plasticity of the hippocampal formation provides a new view of the resilience of this brain region, while emphasizing the importance of stress and sex hormones in the effects of experience on learning and memory. This plasticity puts a new light on the aging process and raises the hope that treatments can be designed to improve hippocampal function during aging and retard or prevent neurodegenerative disorders like Alzheimer's disease. The final Cyberounds® of this series addresses this aspect -- we consider how sex hormones and sex differences play an important role as agents of plasticity and neuroprotection.
Acknowledgment
Research in the author's laboratory on some of the topics discussed in this article is supported by NIH Grants NS07080 and MH41256 and by the Health Foundation (New York), Servier (France) and UCB (Belgium).