Course Authors
Frieda Wolf, M.D.
Dr. Wolf is Assistant Professor of Medicine, Department of Medicine, SUNY-Downstate Medical Center, Brooklyn, New York.
Within the past 12 months, Dr.Wolf reports no commercial conflicts of interest.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Describe the basic components of the immune response; cell-mediated and antibody-mediated immunity
Discuss the commonly used immunosuppressive agents and their mechanisms of action
List the common opportunistic infections in renal transplant recipients.
Among ESRD patients, renal transplant recipients have the best outcomes. Yet, despite their improved survival, these patients face, as a consequence of their treatment, significant challenges -- cardiovascular disease and malignancy. Nevertheless, transplantation is the preferred method of renal replacement therapy for individuals who can tolerate the immunosuppressive medications and who have the greatest probability of rehabilitation.
Acquired Immune Deficiency
ESRD is associated with decreased response to vaccination, impaired cell-mediated immunity, lowered numbers of some T cell subsets, a reduced CD4/CD8 ratio,(2) B cell lymphopenia(3) and an ongoing chronic proinflammatory activation that prolongs survival of lymphocytes.(3) In effect, uremia is an acquired immune deficiency syndrome.
With transplantation, we take individuals who are in fact immune deficient and suppress their immunity even further. The reason for this is that part of our immune system's function is to differentiate the components that belong to our selves from foreign parts. The immune system does this by non specific mechanisms, such as inflammatory reactions, and by specific mechanisms. In the latter, we target specific components of the immune response such as cytokines or antibodies. Our evolving knowledge of immunity and growing experience with immunosuppression has enabled the success of renal transplantation.
Tissue-typing
Our cells have surface receptors that tell our immune system whether they originate from our selves or come from a foreign source. These are called Human Leukocyte Antigens (HLA). Major histocompatibility complex (MHC) molecules are genes expressed on chromosome 6, which encode the HLA system. HLA Class I antigens represent the 'self' identity and are present on all nucleated cells. HLA class II proteins are expressed on the surface of antigen presenting cells, B and T lymphocytes and glomerular endothelial cells, which 'travel' with the allograft into the recipient.
We utilize three subtypes of HLA antigens in transplantation: HLA-DR (class I), HLA-A and HLA-B (class II). Every individual inherits two alleles for each antigen from their parents. These alleles are sequenced and 'matched' by the tissue-typing labs of a transplant center. For each patient, six alleles are sequenced, two for each of three HLA antigens. HLA-DR is of particular importance since it is expressed constitutively on renal tubular cells, T cells and glomerular endothelium. Antigens A and B do not have such a large impact on outcome
Class I and class II HLA bind to CD8 and CD4 receptors, respectively, activating a cascade of cellular events.
The Immune Response
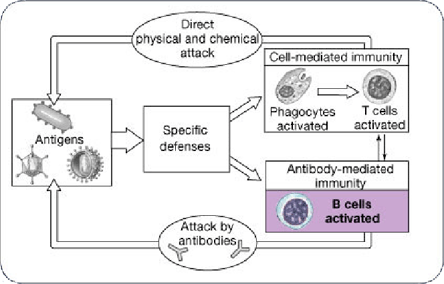
The immune response consists of nonspecific defenses, a cellular component and a humoral component. Nonspecific defenses involve cells that act as phagocytes. These may be neutrophils, monocytes or eosinophils. The phagocytes either lyse the abnormal cell with natural killer cells or activate the membrane attack complex of complement and stimulate inflammation. Specific defenses include T cells which play a central regulatory role in the activation of the immune system.
The Cellular Immune Response
An antigen presenting cell (APC) begins by phagocytizing the foreign particle. It is then 'chopped up' and binds to MHC II proteins on the cell membrane. This begins the cascade of T cell activation. MHC class II proteins activate T helper cells by binding CD4 proteins on their surface.
These helper cells further activate proliferation of subtypes of T cells some of which are other T helper cells, but others are cytotoxic T cells or memory T cells. The 'chopped up' antigen also binds MHC class I proteins on the cell surface, which in turn interact with CD8 molecules on the surface of cytotoxic T cells. T cells are activated via a complex called TCR ( T cell receptor), which is closely associated with CD3 complex.
Activation of T cells ultimately results in expression of genes that regulate cell function via different pathways. One of these pathways is the calcineurin pathway, which is utilized in transplantation.
The T helper cells not only activate T cells but also activate B cells, the other major players of the immune system. These cells are activated both by T cells and by antigens presented to their proteins. Specific antigens stimulate specific antibody production. These antibodies are then placed on the B cell surface and are able to directly recognize the foreign antigenic fragments which bind them.

Figure 1. The Immune Response; Specific Defenses Occur Via Cell-Mediated and Antibody-Mediated Immunity.


The Humoral Component
Antibodies have several roles. They assist in neutralization and opsonization of foreign particles. They prevent adhesion to cell surface molecules. They activate complement cascade and attract phagocytes. Overall, they induce inflammation. This type of immunity is termed humoral because it is mediated by 'humoral' factors.
Immune Memory
Activation of antibody mediated specific immunity is initialized by formation of IgM. After antigen presentation and activation of B cells by a 'new' antigen, plasma cells secrete IgM. After memory B cells are formed, they secrete IgG secondarily. This is termed the secondary immune response. Specific antigens can then be "forever" remembered; their identity is 'recorded' by memory B cells. There are also memory T cells.

Figure 2. Overview of the Integrated Immune Response.
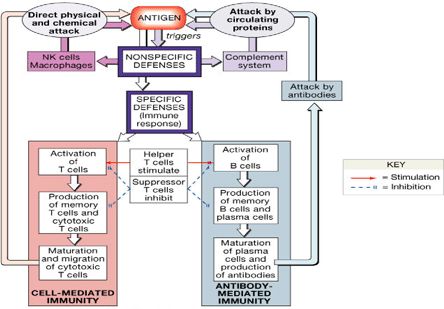

Transplantation is possible only because our knowledge of the immune system enables clinicians to take advantage of the system's primary characteristics and facilitate 'acceptance' of foreign tissue. The primary properties of immunity are:
- Versatility - the immune system is ready to react to new antigens at any time
- Specificity - a specific antigen triggers a specific antibody response
- Memory - immune cells of all types have memory subtypes and they 'hold a record' of the stimulus which induced them
- Tolerance - the system will react to foreign substances but not to 'self.'
Immune Activation and Immunosuppression -- Serial Phases
The T-lymphocyte plays a key role in the early immune events occurring after exposure to an allograft. Ultimately, T-cells regulate B-cell activity leading to a combination of cellular and humoral rejections virtually in all allografts. In kidney transplant patients, this phenomenon is called chronic allograft nephropathy. However, in a small number of anecdotal cases, allografts have been 'accommodated' by a recipient, who stopped taking his immunosuppression, and continue to function. This is termed tolerance.

Figure 3. Events Following T Cell Activation.
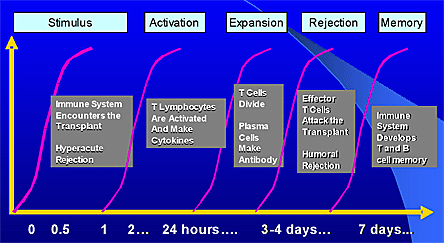
Courtesy of Dr. Martin Zand

Immunosuppression is delivered to organ transplant recipients in serial phases. The first response of the immune system is nonspecific and depends on memory cells, nonspecific phagocytes and inflammation. This response is targeted by steroids, still largely used in transplantation and many other inflammatory diseases.
Rejection
Rejection is the immunologic reaction of our immune system to foreign antigens. In transplantation, hyperacute rejection refers to rapid onset rejection, which occurs immediately because of pre-formed antibodies. Acute rejection may happen at any time during the allograft's life span but most commonly appears in the first 3-6 months post transplantation. Chronic rejection is the term for slow chronic interstitial fibrosis caused by immune activation and/or nephrotoxicity of immunosupression. Chronic rejection, also known as chronic allograft nephropathy, occurs in the majority of allografts and results from alloantigen activation or non-immune causes.
Preformed anti-HLA antibodies, those 'stored' by memory B cells, are lying in wait for the transplant and if allowed to release their antibodies will cause hyperacute humoral rejection. This sequence implies that these B cells have been previously exposed to the antigenic stimulus and have had an opportunity to form antibodies. Hyperacute rejection results in diffuse cellular infiltration, clotting and, ultimately, graft loss.
Hyperacute rejection is very rate today, as we try to measure levels of 'preformed antibodies' and we measure antibodies which are specific to the donor by performing a cytotoxic assay (mixing donor and recipient lymphocytes) immediately prior to the transplant. If this assay is positive, the transplant cannot proceed, since we expect hyperacute rejection to occur.
Maintenance immunosuppression continues for the life of the allograft. The ongoing struggle for the clinician is to determine the overall degree of immunosuppression required to prevent rejection of the allograft without producing the consequences of oversuppression -- infections and malignancy.

Figure 4. The Phases of Immunosuppression.

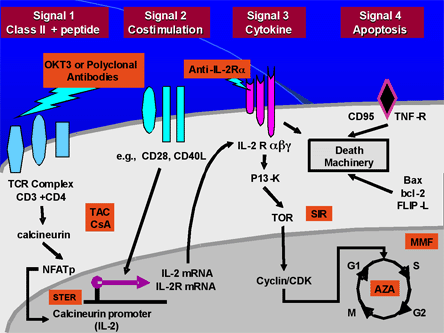
Tac=tacrolimus; CsA=cyclosporine; Ster=corticosteroids; Sir=sirolimus; MMF=mycophenolate mofetil; AZA=azathioprine
Courtesy of Dr. Martin Zand

T-cell activation and proliferation require at least three signals mediated by the interaction with alloantigens. Signal 1 is the first signal in the activation of the IL-2 transcription pathway by a specific antigen and involves the T cell receptor complex (C3+CD4) recognizing the MHC + peptide presented to it by an antigen presenting cell (APC). Costimulation (signal 2) is non-specific and is mediated by interaction between the B7 ligand on the APC and the CD28 ligand on the T cell. This signal is required for full T-cell activation. These two signals activate the intracellular pathway for IL-2 and other growth factor expression.
Signal 3 is induced by IL-2 and other growth factors and leads to cell cycle progression and proliferation. They are activated via the mTOR (mammalian target of rapamycin). The fourth "signal" is programmed cell death -- a natural consequence of T-cell activation.
Induction Therapy
Induction therapy -- treatments designed to reduce anti-HLA antibody titers (e.g., plasmapheresis, IVIg, anti-CD20 antibodies) -- consists of treatment with anti-lymphocyte antibodies administered immediately after transplantation in selected patients. For highly sensitized patients, it may even begin prior to transplantation.
Maintenance immunosuppression is generally required for the life of the allograft including phases characterized by early acute rejection, immune accommodation and, in some cases, late graft failure. During the maintenance phase, overall immunosuppression is reduced, as the risk for acute rejection is diminished and the risk of over-suppression is increased.
For induction therapy, we generally try to block all arms of the immune system, which include non-specific reactions caused by cytokine release and overall inflammation, as well as specific reactions mediated by T or B cell actions.
Corticosteroids remain the mainstay of induction and maintenance immunosuppression and exert their major effects through inhibition of Glucocorticoid Response Elements (GREs), DNA sequences found in cells which produce various cytokines. The binding of steroids to GREs prevents transcription of cytokines such as IL-1, IL-2, IL-6 and TNF-α and INF-γ. This inhibits transcription of cytokines produced by T cells and inhibits function of dendritic cells, antigen presenting cells and migration of monocytes.
Corticosteroids also inhibit translocation of Nuclear Factor-κB, a transcription factor for many cytokines. Thus, corticosteroids reduce macrophage activation, prevent T-cell proliferation, inhibit cytokine production, decrease adhesion molecule expression and alter lymphocyte trafficking by redistributing lymphocytes into lymphoid tissue. They also suppress non-specific inflammatory response such as fever.
The available anti-IL2-receptor antibodies are monoclonal agents that are not lymphocyte-depleting but, instead, arrest lymphocyte function by blocking the effects of IL-2 on its receptor, thus blocking signal 3.

Figure 5. IL-2 Receptor Blockers.
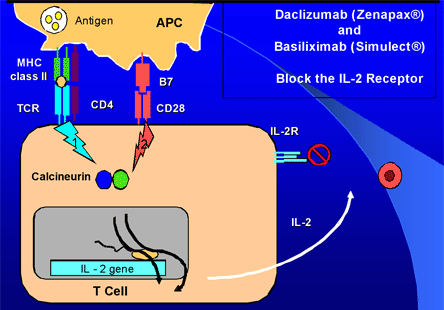
Courtesy of Dr. Martin Zand

Maintenance Therapy
Since the activation of the humoral and cellular components depends on T cell activation, we target interleukin-2 (IL-2), the major cytokine involved in T cell activation and proliferation. The calcineurin inhibitors (cyclosporine and tacrolimus) are the leading players of immunosuppression, binding to intracellular immunophilins, cyclophilin or FK binding protein, respectively.
The calcineurin inhibitor-immunophilin complexes inhibit the phosphatase activity of calcineurin. This blockade, in turn, inhibits key regulatory enzymes [e.g., nuclear factor of activated T-cells (NFAT)] that control transcription of IL-2 and other cytokines. They in effect block signals 1 and 2, by interfering with the effects of the T cell receptor and its costimulatory signal.

Figure 6. Cyclosporine and Tacrolimus: Calcineurin Inhibitors.


Acute Rejection
The greatest risk of acute rejection occurs during the first 6 months following transplantation. To reduce this risk post induction therapy, heavy immunosuppression continues, primarily tacrolimus with mycophenolate mofetil or sirolimus. In high-risk individuals, we add corticosteroids, though they are tapered around the sixth month or eliminated completely because they are highly toxic and especially promote osteoporosis.
Maintenance immunosuppression in the United States relies on tacrolimus and in Europe cyclosporine. Although they both block the actions of the phosphatase calcineurin, they do so through distinct pathways, which result in different side effect profiles and allows some choices in immunosuppression.
Mycophenolate mofetil and sirolimus, the newest agents on the block, work via another pathway. Mycophenolic acid, the active component, blocks the enzyme IMPDH (inosine monosphosphate dehydrogenase), the rate-limiting enzyme in the de novo synthesis of purines. Lymphocytes rely heavily on the de novo pathway rather than the salvage pathway. Thus, mycophenolate mofetil inhibits B and T cell function proliferation and recruitment and has the ability, therefore, to prevent ongoing rejection.
Although sirolimus sounds like tacrolimus (because they have similar chemical structures), it works via a different cellular pathway, atttaching to a silolimus-fk binding protein receptor (FKBP). This ligand activates a regulatory kinase, the target of rapamycin (TOR), which regulates cell division at the G1 to S phase and causes cell-cycle arrest in all dividing cells. These TOR inhibitors can be administered safely along with the calcineurin inhibitors, although they do potentiate calcienuerin nephrotoxicity.
Current therapeutic trends are seen in Figure 7.

Figure 7. Most Common Immunosuppression Regimens US, 2002-04.
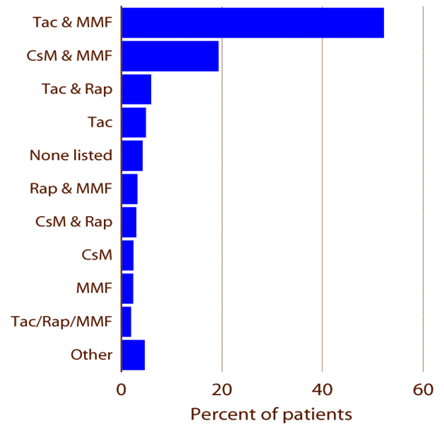

The availability of agents like MMF and sirolimus has allowed corticosteroid avoidance, a good idea given its long-term toxicity. Recent trends in kidney transplantation (Figure 8 below) include dramatic improvements in 1-year graft survival (green line) and a reduction in the incidence of acute rejection during the first post transplant year (yellow line).

Figure 8. Recent Trends in Kidney Transplant Survival.
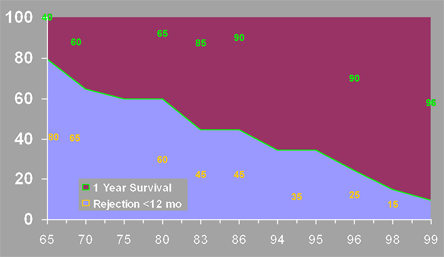
X-axis = year

When outcomes are plotted against the availability of immunosuppressive drugs over time, it becomes evident that outcomes have improved with use of the newer drugs.
With improvement in short-term outcomes, the transplant community is now focused on ways to reduce the long-term effects of chronic maintenance immunosuppression. Furthermore, as the recipient ages, there is also 'waning' of immune function and immunosuppression must necessarily be adjusted to avoid the consequences of oversuppression.
Oversuppression → Viral infections
Though bacterial infections are more common in transplanted individuals than in the normal population, of more concern are the viral infections to which transplanted individuals are predisposed.(2)
Our defense mechanisms against viral infection rely on cellular immunity and cellular pathways which control viral replication. These include the NFκB (nuclear factor kappa B) and the JAK-STAT (Janus family of tyrosine kinases-signal transducers and activator of transcription). Both induction therapy and treatment of rejection block these pathways and, additionally, provoke release of pro-inflammatory cytokines which, ultimately, promote viral replication.
Some viruses may also cause immunomodulation, which increases a recipient's susceptibility to rejection by changing surface antigen expression, particularly MHC antigens. The resultant 'heavier' immunosuppression may stimulate replication of other viruses, which in itself may injure the allograft.
Viruses can, furthermore, be transferred with the allograft, for example Epstein-Barr virus (EBV) or cytomegalovirus (CMV), or may appear through reactivation of 'latent' infection' (e.g., varicella).
Some effects of viruses are caused by direct tissue invasion(6) (e.g., colitis or pneumonitis), while other effects are indirect from release of cytokines and the nonspecific activation of alloreactive T cells.(7) Viral disease is usually seen during the period of most intense immunosuppression -- the first such period occurs one to six months post transplantation.(9)
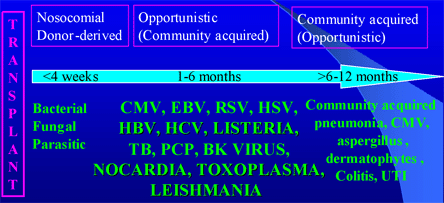
During the initial period after the surgery, nosocomial infections such as C. difficile, vancomycin-resistant enterococci (VRE) or line-related sepsis are most common. The window for opportunistic infections from immunomodulating viruses (CMV, EBV, HBV, HCV, BK virus) begins after induction and persists during the 6-month period post transplantation. The usual temporal sequence is shown in Figure 9 below.

Figure 9. Timeline of Infections Post Transplantation.

Treatment of Viral Infections
Because the temporal sequence for viral infection post transplant is predictable, we use prophylactic medications with immunosuppressive therapy. In the early post transplant period, we administer trimehtoprim-sulfamethoxazole to prevent urinary tract infection (UTI), Pneumocystis carinii pneumonia (PCP) and toxoplasmosis. Antifungal troche is used to prevent mucocutaneous candida. CMV is largely prevented by the use of ganciclovir.
In the late post transplant period (>6 m), most patients are receiving stable and reduced levels of immunosuppression. Community acquired infections are common, however, and approximately 10-15% of transplant recipients have chronic viral infections that manifest. Respiratory viruses remain very important community-acquired pathogens and predispose the patient to the development of bacterial infections and graft rejection. Pneumonia from respiratory viruses, pneumococcus, legoinella and influenza are also seen.

Figure 10. Viral Pathogens in Renal Transplantation.
|
|

HSV
Herpes viruses are very common in the general population and are often reactivated in transplanted individuals. Some viruses are transmitted together such as HHV 6 or HHV 7 with CMV. HHV-6 seems to be a cofactor in CMV infections and may be responsible for the leukopenia seen during infection. Shingles may occur and varicella zoster virus (VZV) may even present as cholangitis. HHV 8 is the herpes virus associated with Kaposi's sarcoma and may be transmitted from the donor and reactivate either in the early period or not until later.
EBV
Exposure to EBV is also very common in the general population. Among immunosuppresed individuals, EBV may be associated with a generalized flu-like syndrome or with the development of B cell non-Hodgkin's lymphoma, particularly in seronegative recipients of seropositive organs. However, some cases of T cell, NK cell and non-EBV-related post transplant lymphoproliferative disease (PTLD) have been described.

Figure 11. EBV with Pharyngeal Exudates.
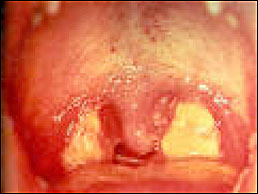
Figure 12. Expansile Plasmacytic Infiltrate in a Case of PTLD.
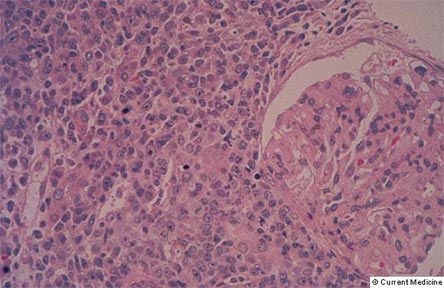

However, most cases of PTLD are the result of Epstein-Barr virus-induced lymphoid proliferation and result in B cell non-Hodgkin's lymphoma. Treatment with increased immunosuppression (for rejection) may be lethal.
CMV
CMV was a persistent problem at the beginning of the cyclosporine era in the 1980s when potent immunosuppression became available. Although CMV may be transmitted from the donor, the virus is common in the general population and risk of exposure increases with age. The risk of disease is highest if the donor is positive and the recipient negative to CMV antibodies but in most cases there is reactivation of previously acquired CMV. CMV may cause tissue-invasive disease during the early post transplant period and later when immunosuppression is reduced.

Figure 13. Photomicrograph with Hematoxylin and Eosin Staining.
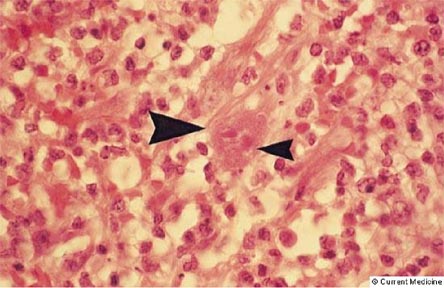
Figure 13 shows typical changes associated with infection caused by CMV. Cells have intranuclear inclusions, halos surrounding the nucleus and intracytoplasmic inclusions. The large arrowhead identifies a CMV-infected cell, while the small arrowhead points to the nuclear halo.

Tissue invasive disease is commonly seen in colitis and pneumonia. Treatment with ganciclovir is effective and is initiated at the same time as reduction in immunosuppression.
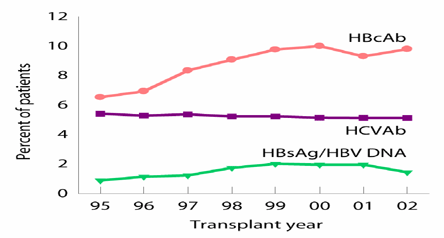
HBV and HCV
Hepatitis viruses are immunomodulating and may reactivate during this time period. They are very common in dialysis patients (prevalence of 20%) and HBV is an indication for isolating a patient. It is generally recommended to treat hepatitis virus prior to transplantation both because the virus may cause disease and precipitate rejection and because the treatment (with pegylated interferon and ribavirin) is nephrotoxic. However, outcome data from HCV+ recipients show that their survival is superior with a transplant than on the waiting list. Nevertheless, the decision to perform transplantation in patients with hepatitis should be very cautious and should include the results from a contemporary liver biopsy. Since mortality is higher for these patients during the first 3-6 months post transplantation,(9) treatment with interferon prior to transplantation is generally recommended.

Figure 14. Pre-Transplant Hepatitis B and C.

BK Virus
BK and JC viruses are polyomaviruses related to human papillomavirus of the papovavirus family. They share a common antigen with a simian virus called SV40. The SV40 antigen is used to detect the presence of the virus, which does not cause disease in the immunocompetent host. In the immunocompromised host, however, BK virus has affinity for renal and uroepithelium. The SV40 antigen can be detected in the serum or urine of affected patients by PCR. It can also be detected by immunohistochemistry staining of transplanted allograft. Since this viral disease is a result of over-immunosuppression, the principal therapeutic strategy is the reduction of immunosuppresion. IVIG may be used if acute rejection is suspected or seen on biopsy and additional immunosuppression seems risky because of the polyoma infection.

Figure 15. BK virus -- Renal Invasion.

Figure 16. BK Virus-SV40 Staining.


Other Pathogens
Anogential and squamous cell cancers, which are more common in immunosuppressed individuals, are caused by human papillomavirus. Parvovirus B19 may also present in the late post transplant period with anemia unresponsive to erythropoietin or with myocarditis. Disseminated histoplasmosis has been reported in transplant recipients as a result of transmission from donor.(10)
Summary
Although transplantation affords longer and better quality of life and is the preferred mode of renal replacement therapy, it is not without its complications. Our expanding knowledge of the immune system -- elucidation of cellular pathways and discovery of drugs that can affect those pathways (e.g., calcineurin inhibitors) -- has enabled and completely altered the face of transplantation. After the advent of calcineurin inhibitors, one-year graft survival increased from 50% to 85%.
Improved survival, however, happened in concert with the rise of opportunistic infections, some of which were newly discovered. Viral infections are part of the risks of being immunocompromised. Some infections follow a predictable timeline and should be considered in the differential diagnosis of any febrile illness or rise in serum creatinine following transplant. A biopsy of the allograft, or other tissue such as colon, may be helpful in differentiating viral disease from rejection. If the appearance of a pathogen occurs during an unexpected period, epidemiologic exposure or oncogenesis must be considered.
Our current clinical challenge is to maintain the balance between effective immunosuppression that prevents allograft rejection with sufficient immune function to prevent opportunistic infections.