Course Authors
Hasan Bit-Shawish, M.D., and John E. Morley, M.D.
Dr. Hasan Bit-Shawish is a subspecialty resident in Geriatrics at St. Louis University and has completed his training as a specialist in Hematology-Oncology. Dr. Bit-Shawish reports no commercial conflict of interest. During the last three years, Dr. Morley has received grant/research support from Vivus, Merck & Co., Upjohn, B. Braun McGaw, Bayer Corp and Nestec, Ltd. He has also served on the Speakers' Bureau for LXN, Organon, Ross, Pharmacia & Upjohn, Glaxo Wellcome, Hoechst Marion Roussel, Searle, Merck & Co., Roche, Bristol-Myers Squibb, Novartis, Pratt, B. Braun McGaw, Pfizer and Parke-Davis.
This activity is made possible by an unrestricted educational grant from the Novartis Foundation for Gerontology.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Develop a protocol approach to the diagnosis and management of anemia in older persons
Discuss the pathophysiology of the anemia of chronic disease
Enumerate the causes of cobalamin deficiency.
Anemia is defined as a hemoglobin below 13 g/dl for men and 12 g/dl for women. The prevalence ranges from 6-30 percent for men and 10-22 percent for women.(1) There is often the impression that anemia of senescence and a mild anemia in the elderly is likely to reflect a physiological rather than pathological process. As a result, the timing of the decision to investigate anemia in an elderly person is often controversial. Some authors suggest that the investigation be postponed until the hemoglobin drops below 10 g/dl since work up of mild anemia may produce a poor yield of identifiable causes. Figure 1 shows the clinical approach to anemia.
Figure 1. A Practical Approach to Anemia in Elderly.

The prevalence of anemia was noted to have increased significantly after 75 years of age.(3) Some studies have observed a greater prevalence of anemia amongst the institutionalized elderly.(2),(3),(4),(5) Sahadaven, in a retrospective study, found that patients from an institutional background tend to present with more severe anemia. The same study demonstrated a lack of classical correlation between RBC size and the specific deficiencies.(2) Only one out of nine iron deficient patients had microcytic RBC; six out of 14 folate deficient patients and three out of five Vitamin B12 deficient patients showed macrocytic pathologies that many elderly patients may be having with the final RBC size being the aggregate of all these conditions. Therefore, we cannot use RBC morphology as a stimulus to investigate deficiency states in elderly. Table I shows pathophysiologic classification of anemia in elderly.
Table 1. Classification of Anemia in the Elderly.
| Hypoproliferative | Ineffective | Hemolytic |
Intrinsic marrow lesion
|
Megaloblastic
|
Immunologic
|
Effects of Age on Erythropoiesis
The regulation of erythropoiesis involves a complex series of interactions and a multitude of regulatory cytokines.(*) There are two forms of erythroid progenitor cells -- the burst-forming unit erythroid (BFU-E) and colony-forming unit-erythroid (CFU-E), the immediate precursor of proerythroblasts.(*)
BFU-E are more primitive and have a high self-replicating capacity. The replicative capacity of the bone marrow far exceeds the life expectancy of the animal. The growth capacity of old stem cells remains characteristically old, even after prolonged self-replication in the bone marrow of a young recipient. In long-term bone marrow culture, the maintenance of hematopoiesis varies inversely with the age of the donor from which the culture was initiated.(6) This fact suggests an intrinsic characteristic of the stem cells that cannot be altered.
It seems that both the age of replicating cells and the microenvironment of the bone marrow are important for replicative capacity. No age related alteration was found in the plasma and erythron iron turnover, red cell survival or the absolute number of marrow erythroid progenitor and differentiated cells.(7)
Experiments showed that, when old animals are exposed to low oxygen pressure, the expected increase in hemoglobin is more variable and tends to be slower than in their young counterparts.(8) The aged erythropoietic system appeared to be more sensitive to external stresses.
From these observations, we conclude that, while basal erythropoiesis is unchanged with aging, reserve capacity is compromised, making the aged animal more susceptible to relatively minor pathologic stress that doesn't impair erythropoiesis in younger animals.
An extensive evaluation of a carefully selected, healthy group of elderly male subjects with a mild anemia revealed no decrease in the level of testosterone.(6) However, the level of DPG was elevated in anemic subjects, indicating that the anemia was pathologic and that O2 delivery to tissue was compromised. It should be noted that more recent studies suggest that free testosterone is decreased in almost every older male and, thus, the finding of no relationship with testosterone needs to be re-evaluated.
The anemia was also associated with a decreased number of CFU-E and marrow normoblasts but no decrease in the number of BFU-E was demonstrated. In addition, a reduction in marrow myeloid elements and circulating neutrophil counts was observed, indicating that anemia was a reflection of a more generalized marrow failure.
Studies showed that erythropoietin response to anemia is not altered with aging and a true anemia does not occur, so other etiologies must be considered for the anemia prevalent among elderly humans.(9) Recent findings, for example, demonstrate that anemia is extremely rare in very affluent and healthy elderly communities.
We can reasonably conclude that anemia is never a normal consequence of advancing age. The aging hematopoietic system is, however, more susceptible to minor stresses so that diseases develop more readily in older as compared with younger people. Table 1 shows a practical approach to anemia in the elderly.
Iron Deficiency Anemia in the Elderly (IDA)
After anemia of chronic disease, IDA is the most common cause of anemia in the elderly. IDA is important to diagnose because appropriate iron therapy may improve symptoms and inappropriate iron therapy may cause clinically important side effects. IDA in the elderly may be a marker for occult gastrointestinal pathology.
Although bone marrow aspiration provides a definitive diagnosis of IDA, the value of less invasive tests of iron stores in general populations has been well established.
Iron binding capacity decreases with aging and is affected by factors such as malnutrition and chronic disease, both of which have a higher prevalence in the elderly. Serum ferritin levels increase with aging and may be elevated by acute and chronic inflammatory conditions.
Guyatt et al studied 1334 patients over 65 years old -- 1259 underwent bone marrow biopsy.(10) Seventy-six percent of the participants were outpatients and 183 were inpatients. The mean hemoglobin level was 9.81 g/dl. Seventy-two percent of the patients had no medical diagnosis other than anemia. In this study, ferritin levels (Table 2) proved to be the most efficacious test for the diagnosis of iron deficiency and its differentiation from anemia of chronic disease.
Table 2. Likelihood Ratios.
| Interval | Number Iron-Deficient | # Not Iron-Deficient | Likelihood Ratio |
| Ferritin | |||
| > 100 | 8 | 108 | |
| > 45 < 100 | 7 | 27 | 0.13 |
| > 18 < 45 | 23 | 13 | 0.46 |
| < 18 | 47 | 2 | 3.12 |
| Total | 85 | 150 | 41.47 |
| Transferring saturation | |||
| > 0.21 | 9 | 55 | |
| > 0.8 < 0.21 | 23 | 70 | 0.28 |
| > 0.05 < 0.08 | 14 | 17 | 0.57 |
| < 0.05 | 38 | 4 | 1.43 |
| Total | 84 | 146 | 16.51 |
| Mean cell volume | |||
| > 95 | 2 | 32 | |
| > 91 - < 95 | 5 | 26 | 0.11 |
| > 85 - < 91 | 16 | 44 | 0.34 |
| > 74 - < 85 | 32 | 42 | 0.64 |
| < 74 | 30 | 6 | 1.35 |
| Total | 85 | 150 | 8.82 |
| Red cell protoporphyrin | |||
| > 0 < 0.75 | 10 | 53 | |
| > 0.75 < 0.1 | 8 | 28 | 0.34 |
| > 1 - < 1.25 | 9 | 21 | 0.51 |
| > 1.25 < 2 | 17 | 24 | 0.77 |
| > 2 | 40 | 24 | 1.26 |
| Total | 84 | 150 | 2.98 |
Red cell distribution width (RDW) added little to the predictive power of serum ferritin. MCV did not influence the estimate of the probability of IDA. Even MCV values of less than 74 were not invariably associated with IDA and in many of the patients with iron deficiency the anemia was not microcytic. Only 6% of those with MCV greater than 95 had IDA. Therefore, a very large MCV can be interpreted as virtually excluding IDA. In this study (Table 3) and, in contrast to previous studies, ferritin values between 18-45 mg/l reflected an increase in the likelihood of IDA. The optimal cut off, in terms of maximizing accuracy, was 45 mg/l and reflects the fact that ferritin levels increase with age. It may also reflect the high prevalence of chronic disease in the elderly.
Table 3. Post-Test Probability of Iron Deficiency Given Varying Pre-Test Probabilities and Results of Serum Ferritin Determinations.
Pre-Test Probability
| Low (5% - 20%) | Intermediate (40% - 60%) | High (80% - 95%) | Study Population (36%) | |
| Serum ferritin result (mg/L) | ||||
| >100 | 0.63-3 | 8-16 | 34-71 | 7 |
| 45-100 | 2-10 | 24-41 | 39-90 | 21 |
| 18 - 45 | 14-44 | 68-82 | 93-98 | 64 |
| <18 | 69-91 | 97-98 | 00-99.9 | 96 |
Patients with serum ferritin more than 100 mg/l can be treated as not having IDA. Bone marrow aspiration is necessary for diagnosis in those with intermediate values. If we use this value, we would diagnose iron deficiency in 21% of the patients and exclude IDA in 49%. Thus, bone marrow aspiration would be required in only 30%.
When unexplained IDA occurs in the elderly patient, it is almost exclusively due to blood loss from the intestinal tract, even if the bleeding is not detected by repeated stool guaiac determination (Table 4).(11)
Epistaxis, abnormal bleeding from the uterus and hematuria are rare but easily recognizable causes of blood loss in elderly patients.(11) Causes of gastrointestinal bleeding in the elderly include drugs and neoplasm.
Table 4. Etiologies of Anemia Associated with Iron-Deficient Erythropoiesis in the Elderly Patient.
Iron deficiency anemia
Anemia of chronic disease and inflammation
|
Angiodysplasia of the large bowel and diverticular disease are frequent causes but they should be considered only after a neoplasm has been excluded. A multifactorial etiology of anemia is very likely in frail, older people with multiple medical problems, such as blood loss, malnutrition, hemolysis and folate deficiency.
Therapy
Oral Iron
Approximately 30 mg of iron is absorbed upon daily administration of 180 mg of elemental iron. Thus, a standard dosage would be 60 mg of elemental iron three times a day between meals to maximize absorption.(12) However, a single dose a day will produce the same effect in the majority of persons with normal absorption. GI side effects, such as nausea, abdominal cramping, epigastric distress, constipation and/or diarrhea, are noted in approximately 15-20% of patients receiving oral iron supplements. These appear to be dose related. Correction of the anemia usually occurs within six weeks. Reaccumulation of iron stores, to approximately 500 mg, takes at least 4-6 months because of both decreased absorption following correction of the anemia and often continued iron losses. Serum ferritin usually normalized upon correction of iron stores.
Parental Iron Therapy
Indications for parenteral iron therapy include malabsorption, intolerance to oral preparations and iron losses exceeding maximal oral replacement.
Sideroblastic Anemia
Sideroblastic anemia occurs in both inherited and acquired forms. Generally, the acquired form is a disease of older adults and is considered a myelodysplastic syndrome. It is characterized by markedly increased ineffective erythropoiesis associated with increased intestinal absorption. In addition, many patients are transfusion dependent and, therefore, are prone to accumulate excess iron from the transfused red blood cells. The sideroblastic anemias, other than the idiopathic forms, may respond to treatment with pyridoxine. Many of the drugs that cause toxic sideroblastic anemias are pyridoxine antagonists. If toxic etiology is suspected, discontinuing the drug or alcohol will often lead to rapid improvement in the anemia. Repeated transfusion may be required to treat severe anemia. A small number of patients will develop progressive bone marrow failure, cytopenias or overt acute myelogenous leukemia. However, most patients will have stable anemia and associated symptoms over many years.
Anemia of Chronic Disease (ACD)
ACD is a frequent event in elderly patients, complicating a variety of disorders, including inflammatory processes, malignant diseases and infections. It was reported as the most common type of anemia in hospitalized patients. Indeed, this anemia occurs in patients suffering from acute infections. Many chronic diseases, such as hypertension and diabetes, are not accompanied by anemia.(28) A major component of ACD is often due to protein energy malnutrition.
Affected patients usually develop normocytic, normochromic anemia that is mild and nonprogressive one to two months after the onset of chronic disease.
ACD is associated with decreased serum iron level, decreased transferrin saturation and normal to increased ferritin. The bone marrow examination reveals that erythroid precursors are, generally, present in normal numbers but the erythroid iron is decreased while the storage iron is increased (Table 5).
Table 5. Differential Diagnosis of Serum Ferritin Levels.
| Serum Ferritin (ng/mL) | Probable Diagnosis |
| < 20 | Iron deficiency |
| 20-50 | Iron deficiency and ACD |
| > 50 | ACD |
There are three mechanisms involved in the development of ACD:
- failure of erythropoiesis
- lack of iron for hemoglobin synthesis and
- decreased RBC survival.
Infection, inflammation or other triggering causes release of IL-1, a cytokine, which is thought to play a major role in the development of this anemia.(13),(14),(15) IL-1 acts on macrophages and induces phagocytosis, which decreases RBC survival. This effect is associated with increased complement, ferritin and IL-1. The effect of IL-1 on hepatocytes results in decreased albumin and transferrin.
The IL-1 effect on granulocytes is due to released lactoferrin, an iron binding protein found in high concentration at the site of inflammation.(16) Lactoferrin is similar in size, shape and affinity for binding iron to transferrin but it does not transfer its Fe to erythroid precursors and, instead, returns it to macrophages of the reticuloendothelial system for storage. As a result of these effects, there will be hypoferremia and an insufficient supply of Fe for erythropoiesis in the bone marrow. The decrease in iron in ACD stimulates apoferritin synthesis which increases intracellular iron and decreases its release, so there is increase of serum ferritin.
It is believed to be a hemolytic component that contributes to the anemia in ACD. RBC survival is mildly decreased (80-90 days). The hemolysis is due to increased phagocyte capacity of the macrophages.
In a study done in 56 patients with ACD over 70 years, erythropoietin levels were significantly higher in anemic patients than in controls.(17) Erythropoietin levels were not significantly different in the three groups of ACD; cancer, infection or inflammatory disease. Similar erythropoietin values were obtained in patients with hemoglobin levels ranging from 85-110 g/l, whereas a significantly higher erythropoietin response (p < 0.05) were obtained for hemoglobin levels less than 85 g/l.
There was no correlation between erythropoietin and albumin, suggesting that the moderate erythropoietin production in ACD was not related to a generalized reduction in serum protein concentrations.(17)
IL-1 can suppress bone marrow activity when systemic inflammatory or infectious processes are active. This may be the main factor responsible for the lack of compensatory marrow changes.
The management of ACD must be tailored to the individual patient and the primary disease process. This makes accurate diagnosis very important. A patient with an acute infection and a mild hypoproliferative anemia can be expected to recover spontaneously as the infection is treated. The same is true for a patient with chronic inflammatory anemia unless the patient is elderly with cardiovascular disease. In this case, judicious transfusion therapy may be necessary to maintain the patient's exercise tolerance and well being.
Megaloblastic Anemia (MA)
Megaloblastic anemia is a descriptive morphologic term that refers to abnormal hematomyelopoiesis, characterized by dyssynchronous nuclear and cytoplasmic maturation in all myeloid and erythroid cell lines.(12) This is the direct result of aberrant DNA synthesis provoked by a single or combined deficiency of either cobalamin (Cbl) or folate. Recent evidence suggests that cobalamin deficiency is much more common in older people than is generally perceived. MA occurs most frequently in patients older than 60 years of age and may be severe.(*) Cobalamin deficiency can cause permanent neurologic disability, such as spinal cord degeneration and mental status changes. If left untreated, it is estimated that 80-90% of deficient individuals will develop neurologic disorders, such as fatigue, ataxia, muscle aches, irritability, paranoia and dementia. However, if therapy is started within three to six months after the onset of symptoms most neurologic deficits will resolve.
Macrocytosis occurs prior to anemia in nearly all subjects with MA. MCV value more than 95 fl should prompt the physician to institute further diagnostic investigation.(17) Figure 2 shows the algorithm approach to evaluate macrocytosis. A normal MCV may, however, be encountered in patients with MA when complicated by a coexistent iron deficiency. Figure 2 shows differential diagnosis of macrocytosis.(29)
Figure 2. Differential Diagnosis of Macrocytosis.
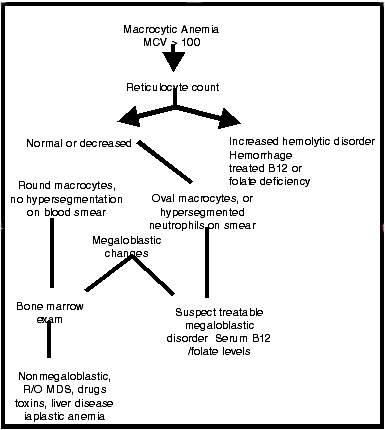
Microscopically, the earliest morphologic sign of MA is the detection of hypersegmented neutrophils (> 5-lobed) neutrophils.(29) The bone marrow changes are identical in both cobalamin and folate deficiency. In all cell lineage, there is a disparity between the nuclear and cytoplasmic maturation. Erythropoiesis and myelopoiesis are ineffective.
Similar morphologic changes occur in aerodigestive tract or uterine cervix which may lead to an erroneous diagnosis of dysplasia or even neoplasia when cytologic smears are viewed. Table 6 demonstrates the causes of cobalamin deficiency.(12)
Table 6. Etiologies of Cobalamin Deficiency.
Inadequate intake (strict vegetarians), Defective absorption
Impaired utilization of cobalamin
|
Animal products are the primary dietary source of cobalamin.(18) Meat contains hydroxy and adenosyl cobalamin and dairy products contain hydroxy and meth cobalamin. The average adult requirement for cobalamin is approximately 1 mg/d. Figure 3 demonstrates enteric processing and absorption of cobalamin. Intrinsic factor (IF) is required for the absorption of cobalamin in the intestine. Each IF molecule binds two molecules of cobalamin and transfers the vitamin to the distal ileum for subsequent absorption.(18) Cobalamin is attached to TCII in the ileum and, subsequently, transported to the liver.
Figure 3. Enteric Processing and Absorption of Cobalamin (CBl).
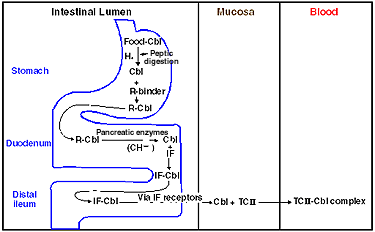
IF = intrinsic factor; R-binder = a cobalophilin with a rapid (compared with IF) electrophoretic mobility; TCII = transcobalamin II.
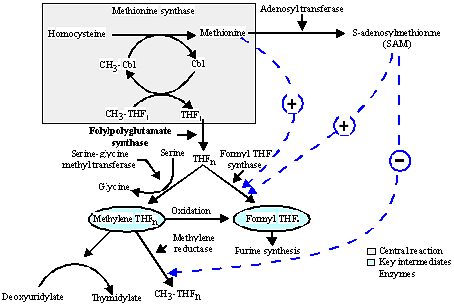
Intracellularly, cobalamin has two enzymatic functions (Figure 4):
- Methylmalonate - succinate isomerization
- Methylation of homocysteine to methionine.
Figure 4. Intercellular Interdependent Cofactor Activity of Cobalamin (CBl) and Folate.
Click to see full sized image
CH3 = methyl group; THF1 and THFn = monoglutamated and polyglutamated forms of tetrahydrofolate.
Cobalamin deficiency leads to intracellular methionine deficiency, which, theoretically, blocks the availability of reduced folate within the cell. This would perturb DNA synthesis by reducing the quantity of thymidylate precursor. Prolonged deficiency of cobalamin in humans ultimately results in defective conversion of propionate to succinyl CoA. In some patients, this results in defective myelin synthesis within the CNS.
The dietary sources of folate are both animal products and leafy vegetables. The adult daily requirement is approximately 200 mg/d and is absorbed in the jejunum.
Intracellularly (Figure 4), folate transfers one C unit at the oxidation levels of methyl C-CH3, methylene-CH2 or formyl (HCO) to facilitate DNA synthesis. To achieve this coenzymatic activity, the folate analogues must be in both a reduced form (THF) and the polyglutamated form (THFn).
In DNA synthesis, the formation of methionine is the central reaction. Both cobalamin and folate are necessary for this reaction.
Neurologic manifestations of cobalamin deficiency were described as early as 1885(19) Involvement of both the CNS and peripheral nervous system cause various clinical manifestations, including paresthesia, loss of deep tendon reflexes, long tract signs, impaired proprioception and vibratory sense, memory deficit and psychosis. The underlying pathologic changes include demyelination and axonal destruction in the posterior and lateral columns of the spinal cord (subacute combined degeneration). On physical examination, decreased vibratory sense is the most common abnormality (88% of cases), while decreased proprioception was noted in 60% of cases in one study. Other signs include ataxic gait, Romberg's sign, limb weakness, spasticity and altered mental status (impaired memory, dementia, depression and psychosis). Paresthesia and/or numbness were the most frequent complaints. During approximately 16% of the neurologic episodes, neither anemia nor macrocytosis was evident.(20)
Patients with pernicious anemia have an estimated risk of gastric adenocarcinoma that ranges from three to five times that in the general population.(21),(22),(23)
A review of laboratory results of 100 patients with confirmed pernicious anemia, based on the presence of IF Abs and Schilling test (or both), found that only 29% had a Hb < 12 g/dl, 17% had HCV of less than 90 fl, 64% had an MCV of more than 100 fl, 9% had WBC less than 4 x 109/L and 12% had platelet counts of less than 150 x 109/L.(18) In the same study, the median serum B12 level was 55 pg/dl, 46% had undetectable levels and 22% had levels that exceeded 100 mg/dl. The Vitamin B12 level predicted neither the degree of anemia nor the MCV.
In a recent study up to 40% of patients with increased values for urinary methylmalonic acid (MMA) had normal serum cobalamin levels and 14% had levels that exceeded 350 pg/ml.(24) These patients were found to have either laboratory or clinical evidence of cobalamin deficiency on subsequent follow up. Therefore, a normal serum cobalamin level does not exclude cobalamin deficiency.
Rarely, certain clinical conditions may be associated with false positive or negative serum cobalamin values (see Table 7).
Table 7. Pitfalls in the Determination of Serum Vitamin B12 Levels.
Conditions associated with falsely normal results
Conditions associated with falsely low results
|
Cobalamin is essential in the folate-dependent conversion of homocysteine to methionine and the folate independent conversion of methylmalonyl-CoA to succinyl-CoA. Therefore, Cbl deficiency will result in the accumulation of both homocysteine and methylmalonyl-CoA (MMA), whereas folate deficiency results in the accumulation of homocysteine only. Serum values of MMA and homocysteine are increased in 95% of patients with Cbl deficiency.
Overall, serum MMA values seem to be more sensitive than serum homocysteine levels in the diagnosis of cobalamin deficiency and the sensitivity is increased by measuring both. Alternatively, measuring the urinary MMA level is less invasive, more practical and, possibly, more sensitive for diagnosing Cbl deficiency than is determination of the serum MMA value.(24) In a recent study of elderly patients, up to 5% of 809 randomly tested persons had increased urinary MMA concentrations. Subsequent studies and treatment responses of those available for follow-up uniformly revealed evidence of Cbl deficiency. Of interest, up to 40% of the patients with increased urinary MMA values had normal serum Cbl values.
Other important tests for pernicious anemia is to detect the presence of IF antibody (type I which blocks the binding of Vitamin B12 to IF and type II which react with the IF binding site to the ileal receptors.(25)) Type I is present in 31-76% of patient with pernicious anemia. IF antibody assay is fairly specific but lacks sensitivity because it is absent in about 40% of patients with PA. Antiparietal cell antibodies are detectable in up to 90% of patients with PA but they are less specific than IF antibodies.
The Schilling test is performed to assess the absorption of orally ingested radiolabeled crystalline cyano-cobalamine (1 mg) without (Stage I) and with (Stage II) IF. The radiolabeled Vitamin B12 is administered orally before or with the flushing parenteral dose of nonradioactive Vitamin B12 (1,000 mg) and a complete 24-hour urine specimen is collected to measure the excreted radiolabeled Cbl which should be less than or equal to 7% of the ingested dose (Table 8).
Table 8. Conditions Associated with Abnormal Schilling Test.
Abnormal first stage and normal second stage
Abnormal results in both stages
|
Treatment
The important aspect of Vitamin B12 therapy is that it should be lifelong. The arbitrary recommended dosage of daily injections of 1,000 mg of Vitamin B12 for the first week followed by weekly injections for the first month and then monthly injections for the rest of the patient's life is commonly used because of the negligible toxicities associated with such therapy.
In a recent study,(26) 38 patients with newly diagnosed Cbl deficiency were randomly assigned to receive CoBL (CyanoCobalamin) as either 1 mg intramuscularly on days 1, 3, 7, 10, 14, 21, 30, 60 and 90 or 2 mg orally on a daily basis for 120 days. After four months of therapy the respective values were 1,005 pg/ml, 169 nmol/L and 10.6 :mol/L in the parenteral group. The higher serum cobalamin and lower serum MMA levels at four months post-treatment in the oral group were significant with P < .0005 and P < .05, respectively.(27)
Within hours after the initiation of CoBL therapy, the patient experiences a subjective sense of improvement with no objective increment in the hemoglobin level. Within the marrow, morphologic changes of megaloblastic maturation revert to normal within 24-48 hours. Reticulocytosis occurs by the third day and peaks at seven days. Coincidentally, the serum iron and potassium levels decrease. The hematologic abnormalities should revert to normal within two months but if they fail to do so an alternative cause should be sought for the anemia. Neurologically, most patients' conditions respond either completely (50%) or partially to treatment; the degree and likelihood of response depend on the pretreatment severity and duration of the neurologic symptoms.
Hemolytic Anemias
Autoimmune hemolytic anemia (AHIA) is the most common cause in elderly patients. The diagnosis is made by the finding of a positive Coumbis test. In younger patients, the etiology of the autoimmune hemolysis is only rarely identified. In the elderly patient, however, the anemia is more likely to be associated with a lymphoproliferative disorder (non-Hodgkins lymphoma or chronic lymphocytic leukemia), collagen vascular disease or drug ingestion. There are two major subcategories of AIHA classified by the temperature at which the autoantibody associates best the red blood cell antigen. Antibodies that are maximally active at 37oC give rise to warm-type AIHA and clinical syndromes that differ from those autoantibodies that are maximally active at 4oC (cold-type AIHA).
Steroids and spleenectomy are usually effective in patients with red cell antibodies of the IgG type. Immunosuppression medication can be used in steroid resistant cases and are usually successful. There is little therapy for cold-type AIHA. It does not respond to steroid therapy, immunosuppressive drugs or splenectomy.
Microangiopathic hemolytic anemia is a disorder of importance in older patients. Fragmentational hemolysis may result when blood flow through small vessels is impeded by the presence of microthrombi or by intrinsic disease of the vessel wall. This is usually associated with severe infections or disseminated neoplasm and presents not only with hemolytic anemia but also a consumptive coagulopathy. The finding of red cell fragmentation thrombocytopenia, a prolonged PTT and a hemosiderinuria should suggest this diagnosis.
Myelodysplastic Syndromes (MDS)
MDSs are common in the elderly patient. They are bone marrow stem cell disorders that lead to ineffective and disorderly hematopoiesis. They manifest as irreversible quantitative and qualitative defects of hematopoietic cells caused by abnormal division, maturation and production of erythrocytes, granulocytes, monocytes and platelets. The disorder presents with macrocytosis or a dimorphic red cell morphologic pattern (a microcytic subpopulation of red cells with the remaining cells being normocytic).
The median age at which MDS is diagnosed is between 60-75 years. Signs and symptoms are nonspecific and generally relate to the blood cytopenias. Fatigue, weakness and malaise are common. A small percentage of patients have spleenomegaly, most commonly those with CMML. Hepatomegaly is rare. The bone marrow has features suggesting a DNA synthetic disturbance or abnormality in iron distribution. The features generally used to define an MDS include various combinations of blood cytopenias, ineffective hematopoiesis, dyserthropoiesis, dysgranulopoiesis, dysmegakaryopoiesis and increased myeloblasts. Bone marrow is hypercellular or normocellular, sometimes hypocellular. Myelofibrosis is occasionally observed, most often in therapy related MDS. The most important findings in the bone marrow are the dysplastic changes and, in some cases, increased hyeloblasts. The common chromosome abnormalities seen in MDS are trisomy loss of the long arm of chromosome 5, 7, 8 or 9 monosomy. The etiology is unknown.
The treatment of MDS in elderly patients is different from that of young patients as they are not candidates for bone marrow transplant and do not tolerate intensive cytotoxic treatment. Treatment should be individualized and tailored to the patient's clinical situation. Options to be considered include observation, low-dose chemotherapy, recombinant erytropoietin or colony-stimulating factor that can increase circulating formed blood elements and cis-retinoic acid, which has been shown to facilitate hematopoietic cell differentiation. High dose chemotherapy should be used with caution. Effective, safe therapy for the patient with MDS has yet to be developed.
Conclusion
Anemia is common in older persons. The key to an appropriate diagnosis is to utilize the reticulocyte index. The role of low free testosterone and protein energy malnutrition in the anemia of chronic disease of older persons requires further investigation.